VASCADE MVP®
Safe. Simple. Proven.
VASCADE MVP® venous vascular closure system provides rapid hemostasis for electrophysiology (EP) procedures, eliminating the need for manual compression and enabling patients to get up earlier. VASCADE MVP system is easy to use, and has demonstrated 0% major complications* in 1,223 patients in 5 EP clinical trials.1-5 In the AMBULATE pivotal trial,1 VASCADE MVP system has been proven to reduce time to ambulation by 64%, improve patient satisfaction by 63% and reduce opioid use post procedure by 58%. VASCADE MVP system is FDA approved for same day discharge and for use in multi-stick, multi-limb cardiac ablation procedures.6 Watch the video
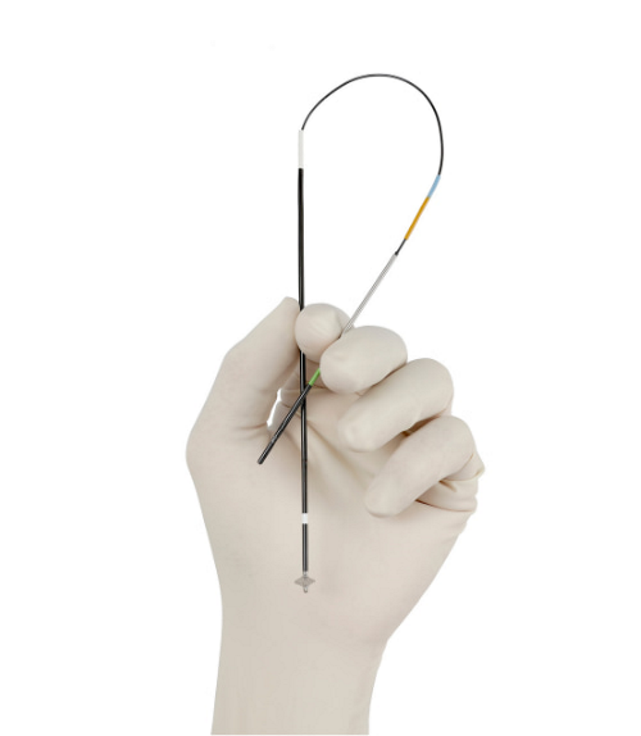
VASCADE MVP® System
Make a selection to learn more
VASCADE MVP® System
0% major complications in 1,223 patients in 5 EP clinical trials1-5
Ordering Information
|
AMBULATE Trial
The AMBULATE Clinical Studies have demonstrated 0% major complications* in 1,223 total patients studied in 5 EP clinical trials, including 4 trials studying same day discharge paroxysmal and persistent AF ablation patients.3-5
The impact of early ambulation using the VASCADE MVP® system is that every stakeholder has the potential to accomplish their specific goals and objectives. Potential goals could be same day discharge, patient satisfaction, opioid reduction, urinary catheter reduction, greater throughput, managing limited bed space and lowering overall costs.

Designed for EP procedures
Ordering Information
|
AMBULATE Trial
The VASCADE MVP system is FDA approved for same day discharge as well as for use in multi-stick, multi-limb cardiac ablation procedures6 and is the only extravascular closure device to offer collagen with a dual method of action, mechanical and physiological.9 Using a single operator, the VASCADE MVP system is simple and easy to use and leaves no sutures or other materials left behind in the vessel. The bioabsorbable thrombogenic plug expands by 13X to fill the tissue tract.** The VASCADE MVP system allows for re-access, after 30 days, for repeat ablation procedures.
.jpg)
Proven by EPs
Ordering Information
|
AMBULATE Trial
VASCADE MVP® venous vascular closure system has been proven in the prospective, multicenter randomized AMBULATE Clinical Trial,1 demonstrating a 3.9 hour (64%) reduction in median time to ambulation as well a 63% improvement in patient satisfaction and a 58% reduction in opioid use post ablation, when compared to manual compression. The VASCADE MVP system has also demonstrated same day discharge success in the retrospective and prospective multicenter AMBULATE Same Day Discharge Clinical Studies,4, 5 where 91.2% of patients were sent home the same day using the VASCADE MVP system.4,5 In addition, 99.7% of VASCADE MVP same day discharge patients had no intervention during follow up7 and there were zero (0%) major complications.8


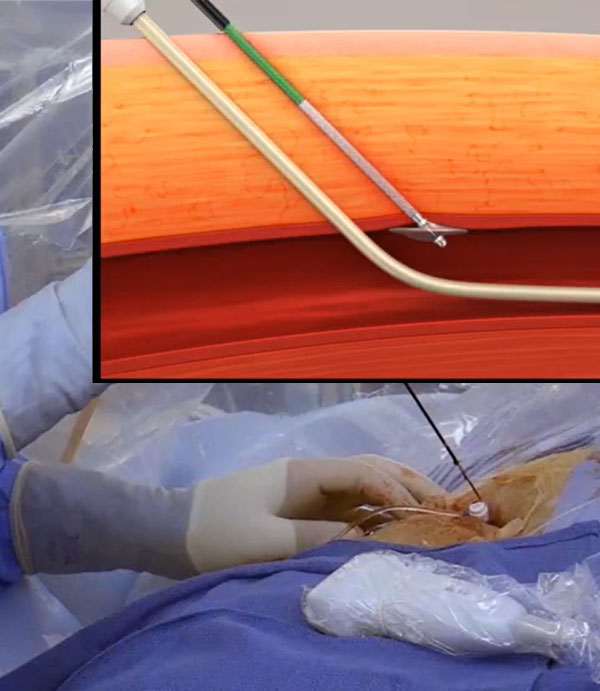
Elegantly Simple and Easy to Use
• FDA-approved for same day discharge and multi-stick, multi-limb access following cardiac ablations6
• Dedicated to mid-bore 6-12F ID (Max 15F OD) multiaccess venous closure
• Single operator
• No sutures or material left in the vessel
• Extravascular design
• No permanent or intraluminal implants
• Bioabsorbable and thrombogenic collagen plug
• Expands to fill tissue tract
Related Training & Resources
Back to top
Webinars

Resource Center
Featured Resources View all resources
Instructions for Use
The VASCADE MVP® Venous Vascular Closure System (VVCS) is intended to seal the femoral vein access site(s) by delivering a resorbable collagen patch, extra-vascularly, at the venotomy site to aid in achieving hemostasis.
VASCADE MVP® Brochure
Our VASCADE MVP system provides rapid hemostasis for electrophysiology procedures, eliminating the need for manual compression and enabling patients to get up and moving earlier.
AMBULATE Trial
This prospective, multicenter, randomized study compared the efficacy and safety of VASCADE MVP® to manual compression for closing multiple access sites after catheter-based EP procedures.
AMBULATE Same Day Discharge Clinical Studies
This prospective, multicenter single arm registry evaluates procedural outcomes using VASCADE MVP® following atrial fibrillation interventions for patients who are discharged the same day.
Ordering Information
| Model Number | Description | Order Quantity |
| 700-500DX-05U | 5 French (Inner Diameter) | 1 Box (5 devices per box) |
| Model Number | Description | Order Quantity |
| 700-580I-05U | 6/7 French (Inner Diameter) | 1 Box (5 devices per box) |
| Model Number | Description | Order Quantity |
| 800-612C-10U | 6-12 French (Inner Diameter) | 1 Box (10 devices per box) |
| Product Name | Model Number | Description | Order Quantity |
| CATALYST II | 500-580C-10U | 5-7 French | 1 Box (10 devices per box) |
| CATALYST III | 600-580CP-10U | 5-7 French | 1 Box (10 devices per box) |
*Major venous access site closure-related complications through the follow-up period
** Data on file at Haemonetics
1. Natale A, et al. Venous vascular closure system versus manual compression following multiple access electrophysiology procedures: The AMBULATE Trial. JACC Clin Electrophysiol 2020; 6(1):111-124.
2. Al-Ahmad A, et al. Results from the prospective, multicenter AMBULATE-CAP trial: Reduced use of urinary catheters and protamine with hemostasis via the mid-bore venous vascular closure system. VASCADE MVP following multi-access cardiac ablation procedures. J Cardiovasc Electrophysiol 2021. 32(2): 191-99.
3. AMBULATE Same Day Discharge Registry Retrospective Study: NCT04538781.
4/5. Eldadah ZA, et al. Same-day discharge following catheter ablation and venous closure with VASCADE MVP: A post-market registry. J Cardiovasc Electrophysiol 2022. https://doi. org/10.1111/jce.15763. NCT04203329.
6. Catheter-based cardiac ablations requiring two or more venous access sites within the same limb. See VASCADE MVP IFU 3972 Indications for Use.
7. Venous access site closure-related complications through 15-day follow up.
8. Major venous access site closure-related complications through 15-day follow up.
9. IFUs of commercially available venous vascular closure devices: VASCADE MVP®, MYNXGRIP®, Perclose ProGlide™ and Perclose ProStyle™. As of 27 Jul 2023.
 Select Language
Select Language  English (Other Regions)
English (Other Regions)  French
French  German
German  Italian
Italian  Russian
Russian  Spanish
Spanish 
