VASCADE®
Safer. Simple.
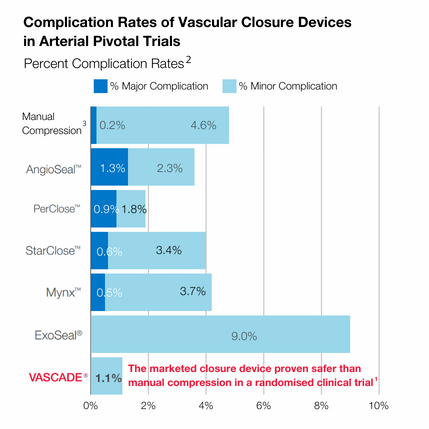
VASCADE® vascular closure system is Haemonetics' next-generation femoral arterial and venous closure system to provide rapid haemostasis after an endovascular procedure. The marketed closure device is proven safer than manual compression in a randomised clinical trial.1
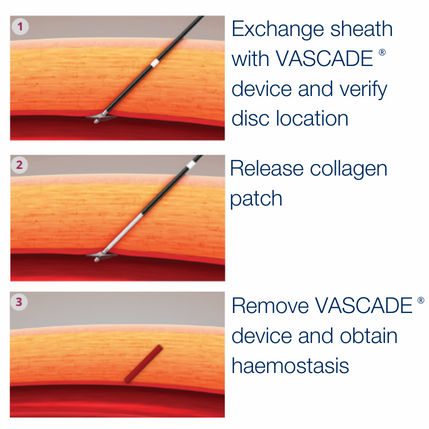
With an elegantly simple 3 step deployment a thrombogenic resorbable extravascular collagen patch is placed to enable rapid haemostasis while minimising complications.

VASCADE® System
Make a selection to learn more
VASCADE® System
Close confidently - helps reduce complications1
Ordering Information
|
RESPECT Trial
The VASCADE® vascular closure system is a fully integrated, extravascular, bioabsorbable femoral access closure system that is easy to use, leaves no permanent components behind, and has demonstrated safety and efficacy in a wide range of patients.1 The system combines the proven collapsible disc technology and a thrombogenic resorbable collagen patch in an integrated design. For patients and healthcare providers, the VASCADE system enables rapid haemostasis while minimising complications.1
Elegantly simple, mechanical and physiological haemostasis
Ordering Information
The VASCADE® vascular closure system is an extravascular closure device providing collagen and a dual method of action, mechanical and physiological. The collagen patch creates a tamponade as a result of rapid expansion of the implant in the presence of fluid. This action ensures the tissue tract is sufficiently filled and provides mechanical haemostasis. The natural thrombogenic property of collagen accelerates coagulation to produce physiological haemostasis.

.jpg?as=0&w=600&hash=25AF502B340D8105ED00F5E7B5608003)
Simple and easy to use
✓ Single operator
✓ Indicated
• For use in 5 and 6/7F 12 cm introducer sheathsa
• For femoral arterial or venous access site closure4
• Reduces time to haemostasis and ambulation4,b
• To reduce time to discharge eligibility4,b
✓ 2 mechanisms of action
• Mechanical
• Physiological
✓ Extravascular design
• No permanent or intraluminal implants
• No sutures or material left in vessel
✓ Bioabsorbable and thrombogenic collagen patch
• Expands to fill tissue tract
aOverall length of the sheath (including the hub) needs to be less than 15 cm
bCompared to manual compression
Resource Centre
Find the information you need as you browse through helpful, on-demand information to aid in your understanding of our vascular closure solutions.
Featured Resources View all resources
Instructions for Use
Intended to seal the femoral arterial or venous access site at the end of an endovascular procedure by delivering a resorbable collagen patch, extravascularly, at the vessel puncture site to aid haemostasis.
VASCADE® Brochure
The VASCADE system combines our collapsible disc technology and a thrombogenic resorbable extravascular collagen patch to enable rapid haemostasis while minimising complications.
RESPECT Publication
The RESPECT trial was aimed at evaluating safety/efficacy of a new extravascular closure system in diagnostic (Dx) and interventional (Ix) procedures performed through 6 or 7 F introducer sheaths.
ANTEGRADE-PVD Publication
This study evaluated short-term outcomes when sealing antegrade femoral puncture sites with an extravascular site-closure system after peripheral endovascular procedures.
Ordering Information
| Catalogue Number | Description | Order Quantity |
| 700-500DX-05E | 5 French (Inner Diameter) | 1 Box (5 devices per box) |
| Catalogue Number | Description | Order Quantity |
| 700-580I-05E | 6/7 French (Inner Diameter) | 1 Box (5 devices per box) |
| Catalogue Number | Description | Order Quantity |
| 800-612C-10E | 6-12 French (Inner Diameter) | 1 Box (10 devices per box) |
2. Data for the chart is from U.S. FDA IFUs and includes both Interventional and Diagnostic treatment arms. All Intention To Treat patients and events are included in the chart. As complication rate data are based on a cross-trial comparison and not head-to-head clinical trials, the data may not be directly comparable due to differences in study protocols, conditions and patient populations.
3. Complication rates for manual compression presented are based on the composite of manual compression complication rates included in the IFUs of the devices presented.
4. Please consult product labels and instructions for use for indications, contraindications, warnings, precautions and adverse events. See VASCADE® EU IFU 5685 Instructions for Use.
Not all products are available in all markets.
 Select Language
Select Language  English (United States)
English (United States)  English (Canada)
English (Canada)  French
French  German
German  Italian
Italian  Japanese
Japanese  Russian
Russian  Spanish
Spanish  Chinese
Chinese 
