VASCADE®
Safe. Simple. Guaranteed.
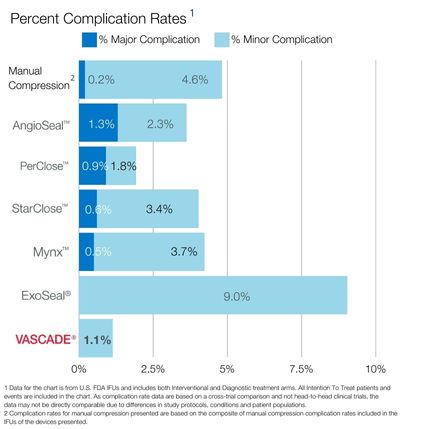
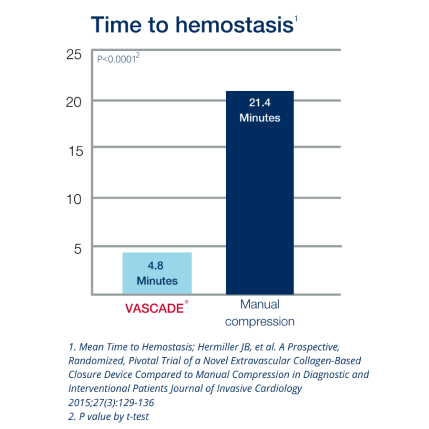
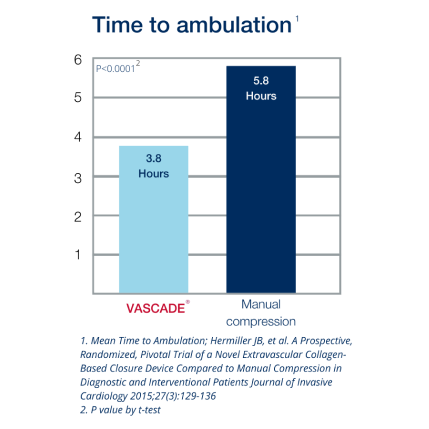
VASCADE® vascular closure system provides rapid hemostasis for interventional and diagnostic procedures and is the only closure device proven safer than manual compression in a randomized clinical trial1 with zero major complications.2 The VASCADE system works by allowing your physician to deliver a collagen patch at the puncture site in the tissue surrounding the vessel. The precise placement of the collagen is accomplished by placing a small collapsible mesh disc against the inside of the vessel wall, which provides temporary hemostasis. Watch the video

VASCADE® System
Make a selection to learn more
VASCADE® System
Rapid hemostasis for interventional and diagnostic procedures
Ordering Information
|
RESPECT Trial
The VASCADE® vascular closure system is a fully integrated, extravascular, femoral access closure system that is easy to use, leaves no permanent components behind, and has demonstrated safety and efficacy in a wide range of patients. The system combines our proven collapsible disc technology and a thrombogenic resorbable collagen patch in an integrated design. For patients and healthcare providers, VASCADE enables rapid hemostasis while minimizing complications.
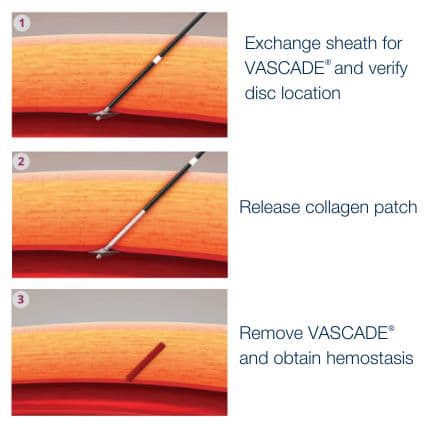
Elegantly simple, mechanical and physiological hemostasis
Ordering Information
|
Watch the video
The VASCADE® vascular closure system is the only extravascular closure device to offer collagen and a dual method of action, mechanical and physiological. The collagen patch provides a tamponade as a result of rapid expansion of the implant in the presence of fluid. This action ensures the tissue tract is sufficiently filled and provides mechanical hemostasis. The natural thrombogenic property of collagen accelerates coagulation to produce physiological hemostasis.
Sharing Risks by Sharing Costs™
Ordering Information
|
Performance Guarantee
Because Haemonetics® is so committed and confident in VASCADE's ability to safely and rapidly achieve hemostasis, we offer a unique value-based purchasing program called the VASCADE® Performance Guarantee. In the event of a qualified complication as the result of VASCADE, Haemonetics will provide financial reimbursement to your hospital or practice. It’s simple, we’re dedicated to exceptional outcomes for you and your patients. We stand behind our products based on proven safety and efficacy published in peer-reviewed clinical studies.
.jpg?as=0&w=600&hash=25AF502B340D8105ED00F5E7B5608003)
— Proven safety & efficacy —
Benefits of using the VASCADE® System
• Simple-to-use technique with no sheath exchange to easily deploy collagen
• Unique design maintains collagen patch position during sleeve retraction and device removal
• Disc technology efficiently locates arteriotomy or venotomy for precise placement and release of pre-loaded patch
• Proven technology that has been used in more than 500,000 procedures
• Fully-integrated femoral access closure system

Webinars

Resource Center
Featured Resources View all resources
Instructions for Use
Intended to seal the femoral arterial or femoral venous access site at the end of an endovascular procedure by delivering a resorbable collagen patch, extravascularly, at the arteriotomy or venotomy site to aid hemostasis.
VASCADE® Brochure
The VASCADE system combines our collapsible disc technology and a thrombogenic resorbable extravascular collagen patch to enable rapid hemostasis while minimizing complications.
RESPECT Publication
The RESPECT trial was aimed at evaluating safety/efficacy of a new extravascular closure system in diagnostic (Dx) and interventional (Ix) procedures performed through 6 or 7 F introducer sheaths.
ANTEGRADE-PVD Publication
This study evaluated short-term outcomes when sealing antegrade femoral puncture sites with an extravascular site-closure system after peripheral endovascular procedures.
Ordering Information
| Catalog Number | Description | Order Quantity |
| 700-500DX-05U | 5 French (Inner Diameter) | 1 Box (5 devices per box) |
| Catalog Number | Description | Order Quantity |
| 700-580I-05U | 6/7 French (Inner Diameter) | 1 Box (5 devices per box) |
| Catalog Number | Description | Order Quantity |
| 800-612C-10U | 6-12F Inner Diameter (15F maximum outer diameter) | 1 Box (10 devices per box) |
| Catalog Number | Description | Order Quantity |
| 800-1012XL-10U | 10-12F Inner Diameter (15F maximum outer diameter) | 1 Box (10 devices per box) |
2. Hermiller JB, et.al. A Prospective, Randomized, Pivotal Trial of a Novel Extravascular Collagen-Based Closure Device Compared to Manual Compression in Diagnostic and Interventional Patients Journal of Invasive Cardiology 2015;27(3):129-136.
 Select Language
Select Language  English (Canada)
English (Canada)  English (Other Regions)
English (Other Regions)  French
French  German
German  Italian
Italian  Japanese
Japanese  Russian
Russian  Spanish
Spanish  Chinese
Chinese 
